News and Announcements
World-First FDA Approval for EBR Systems’ Leadless Cardiac Device Opens US$3.6bn Market
- Published April 22, 2025 3:47AM UTC
- Publisher Wholesale Investor
- Categories Capital Insights, Landing, Trending
As global demand for advanced cardiac therapies escalates, driven by ageing populations and increasing heart failure prevalence, medical technology companies are racing to offer innovative solutions that circumvent the risks and limitations of traditional treatments.
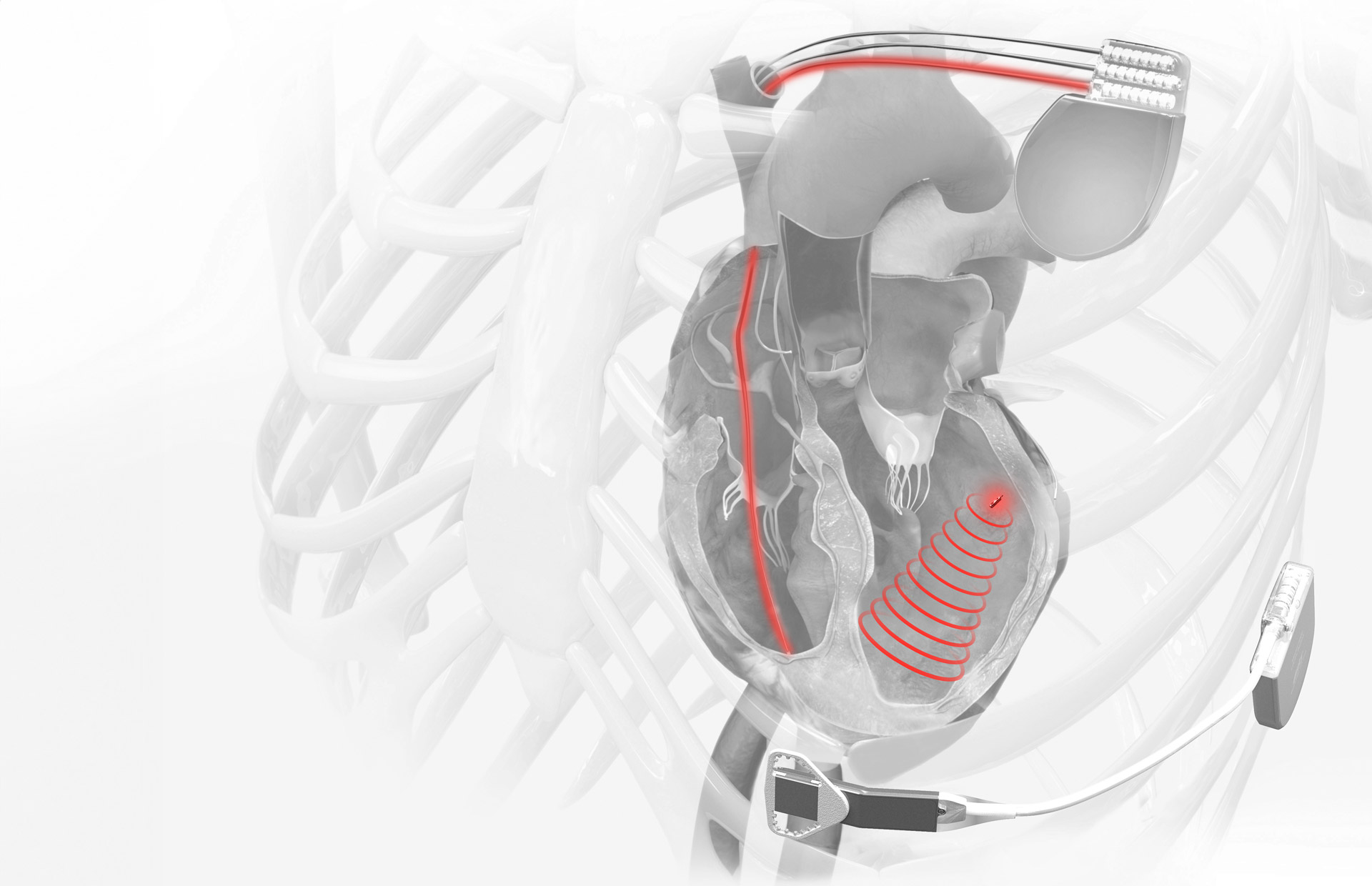
Against this backdrop, Silicon Valley-based EBR Systems (ASX: EBR) has achieved a landmark victory, securing US Food and Drug Administration (FDA) approval for its groundbreaking Wireless Stimulation Endocardially (WISE) Cardiac Resynchronisation Therapy (CRT) system—the world’s first leadless solution for left ventricular pacing. The approval, announced on April 14, marks a critical milestone for both the company and the broader medical technology landscape.
The WISE CRT System addresses a significant unmet medical need by eliminating the necessity for pacing leads—traditionally the weakest link in cardiac pacing systems due to their susceptibility to complications, reliability issues, and failures. “Securing FDA approval for the WISE CRT System is a transformative moment, marking our transition from clinical development to commercialisation,” said John McCutcheon, President & CEO of EBR Systems. “With FDA approval in hand, we are well-positioned to deliver real impact to patients and service a substantial unmet need.”
The device specifically targets patients previously considered untreatable or high-risk due to failed or contraindicated conventional CRT procedures. This category alone represents an immediate addressable market estimated at approximately US$3.6 billion in the US, indicating substantial growth potential for EBR Systems. The FDA-approved indications include adult patients who have an existing right ventricular pacing system but are unsuitable for conventional CRT due to anatomical constraints, previous lead implantation failures, or high-risk upgrade concerns.
“This approval is a significant leap forward,” industry analysts suggest, highlighting the strategic advantage for EBR Systems in the fast-growing cardiac resynchronisation market. CRT is well-established as a critical treatment modality for heart failure, proven to reduce hospitalisations and improve survival rates. Yet, a notable proportion of patients have historically been excluded due to lead-related limitations. EBR’s leadless solution now expands therapy access to these previously underserved patient populations.
Commercialisation of the WISE CRT System will follow a phased approach. An initial limited market release is scheduled to commence later this year, targeting high-volume clinical centres involved in prior clinical trials to gather crucial early-user insights. Full-scale commercial distribution is anticipated by early 2026. Concurrently, EBR Systems will conduct a mandatory FDA post-approval study enrolling 320 patients over five years to ensure ongoing safety and efficacy monitoring.
Financially, the FDA’s Breakthrough Device Designation positions EBR favourably, enabling the company to benefit from significant reimbursement schemes—including the New Technology Add-on Payment (NTAP) and Transitional Pass-Through Payment (TPT)—expected to come into effect from October 2025. These reimbursements will cover the selling price of the WISE CRT System, substantially enhancing its commercial viability.
As EBR gears up for commercial rollout, CEO John McCutcheon emphasised their strategic focus: “We’re expanding our team, strengthening training programs, and partnering closely with hospitals to streamline implantation workflows, ensuring clinicians are fully supported from day one.”
EBR’s success may trigger heightened competition among major cardiac pacing companies, notably Medtronic and Abbott, whose own leadless pacing solutions, Micra and Aveir, respectively, may soon intersect with the WISE CRT System.
Looking forward, EBR Systems’ trajectory appears promising. This FDA approval not only positions EBR as a leading innovator in cardiac therapy but also signifies a paradigm shift in how clinicians manage complex heart failure cases, potentially reshaping industry standards and patient expectations.
Congratulations to EBR Systems on their groundbreaking FDA approval for the WISE CRT System, a monumental achievement in leadless cardiac therapy. This milestone marks a transformative step in addressing critical unmet medical needs and expanding treatment access for heart failure patients.
Trending
Backed By Leading Investment Groups and Family Offices







